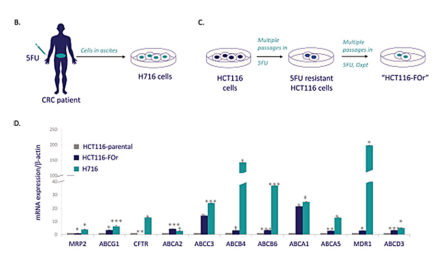
Kristin A. Anderson1,2,*, Andreas S. Madsen3,*, Christian A. Olsen3, and Matthew D. Hirschey1,2,4,**
1 Duke Molecular Physiology Institute and Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27701
2 Department of Pharmacology & Cancer Biology, Duke University Medical Center, Durham, NC 27710
3 Center for Biopharmaceuticals and Department of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen, Universitetsparken 2, 2100, Copenhagen, Denmark
4 Department of Medicine, Duke University Medical Center, Durham, NC 27710
Abstract
NAD+ is a dinucleotide cofactor with the potential to accept electrons in a variety of cellular reduction-oxidation (redox) reactions. In its reduced form, NADH is a ubiquitous cellular electron donor. NAD+, NADH, and the NAD+/NADH ratio have long been known to control the activity of several oxidoreductase enzymes. More recently, enzymes outside those participating directly in redox control have been identified that sense these dinucleotides, including the sirtuin family of NAD+-dependent protein deacylases. In this review, we highlight examples of non-redox enzymes that are controlled by NAD+, NADH, or NAD+/NADH. In particular, we focus on the sirtuin family and assess the current evidence that the sirtuin enzymes sense these dinucleotides and discuss the biological conditions under which this might occur; we conclude that sirtuins sense NAD+, but neither NADH nor the ratio. Finally, we identify future studies that might be informative to further interrogate physiological and pathophysiological changes in NAD+ and NADH, as well as enzymes like sirtuins that sense and respond to redox changes in the cell.
Keywords
Sirtuins; Nicotinamide adenine dinucleotide; Metabolism; Mitochondria; Redox